Looking for Yocon Biology's GMP grade vitrified cell cryopreservation medium
- product advantages and performance
Dr. Daly, a biologist at the famous Whitehead Biomedical Research Center, said: "The 20th century was the era of drug therapy, but the 21st century is the era of cell therapy."
In order to achieve the purpose of cell therapy, cell cryopreservation is one of the most critical links in cell culture. Cell cryopreservation is an important technical means for cell culture, introduction, and preservation and ensuring the smooth progress of experiments. In the establishment of cell lines and lines, it is very important to freeze the original cells in time.
The efficiency, efficacy, and manufacturing process of the final therapeutic preparation of cell therapy are directly affected by cryopreservation, and the safety of cell preparations is also directly related to the components of the cell cryopreservation medium. Cell cryopreservation fluids required for quality and effectiveness are critical to the successful commercial production of cell therapies.
Yocon Biology's GMP-grade vitrified cell cryopreservation medium is a cell cryopreservation medium with defined components developed to meet the drug declaration of cell preparations. In this article, we will fully demonstrate the excellent performance of this cell cryopreservation medium from the four major advantages of the product and experimental data.
Product Name | Quantity | Catalog No. | Storage |
GMP Grade Vitrified Cell Freezing Medium | 100mL/bottle | NC1011 | Store at room temperature, 12 months |
Product Description:
A cell cryopreservation medium with clear chemical composition, which is specially used for cryopreservation of mesenchymal stem cells. Youkang Bio GMP grade vitrified cell cryopreservation medium uses vitrification cryopreservation technology. The cryopreservation medium solidifies at ultra-low temperatures, and the formed glassy solid preserves the normal molecular and ion distribution in the liquid state. After a certain period of time to balance, the cell volume can vary. After dehydration, the cells are instantly vitrified after being put into liquid nitrogen, and stored without the formation of ice crystals. Without cryoprotectants such as DMSO and protein, the cells can be prevented from being pierced by ice crystals, and high viability can be achieved during recovery.
Product advantages:
1. The ingredients are clear
This product is protein-free, animal-free, and does not add serum or serum substitutes. All ingredients are clear, in line with the principle of clear ingredients in the clinical evaluation guidelines, and are suitable for users who have high requirements for cell cryopreservation.
2. Safe and effective
This product does not contain DMSO, serum, and other animal-derived components, has no cytotoxicity, and has stronger protection for cells. The cell recovery rate is stable above 90%, and the cell recovery rate is higher than that of traditional cryopreservation mediums.
3. Easy to operate
Different from the traditional cryopreservation method, using this product for cell cryopreservation does not require programmed cooling. It can be directly placed at -80°C overnight and stored in liquid nitrogen for a long time. The operation is easier and the production efficiency can be greatly improved.
4. Cost saving
Since this product does not contain protein, it can be transported and stored at room temperature, and the cost of transportation and storage is greatly reduced.
Performance Display:
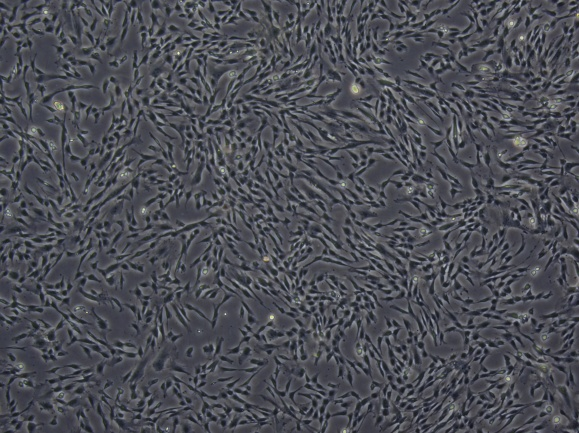
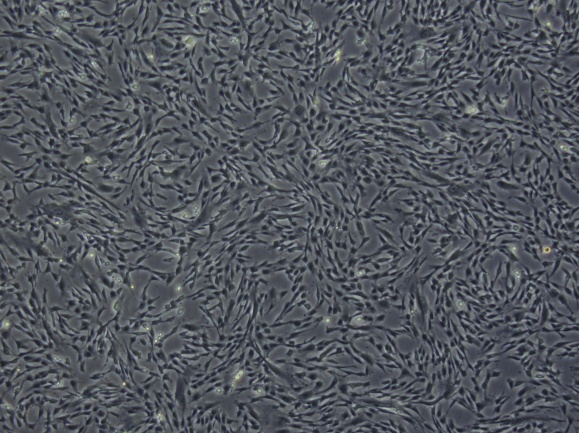
Cell recovery state
P5 |
P6 |
The cells revived with GMP-grade vitrified cell cryopreservation medium were passaged at 8000/cm2and the confluence reached 90% within 72 hours. The cells adhered well, with uniform growth direction and full shape.
Verification 1: The cell recovery rate of Youkang’s GMP-grade vitrified cell cryopreservation medium can be stably maintained at a level close to 100%, and the cell recovery rate is slightly higher than that of DMSO cryopreservation medium, and much higher than that of imported finished product cryopreservation medium.
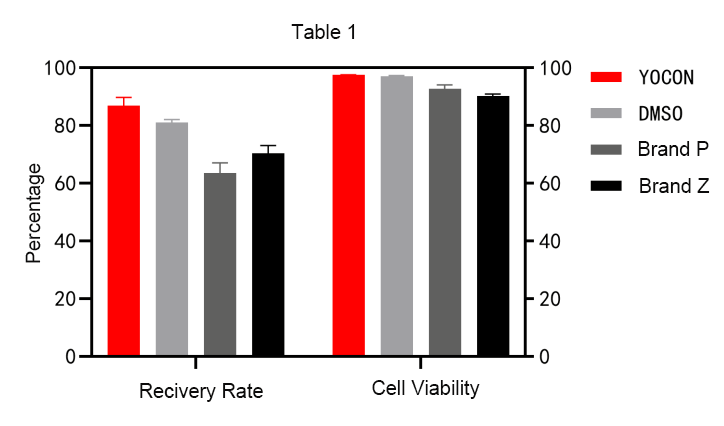
Verification 2 : The cell recovery apoptosis rate of Yocon Biology GMP grade vitrified cell cryopreservation medium is lower than that of DMSO cryopreservation medium and imported finished product cryopreservation medium.
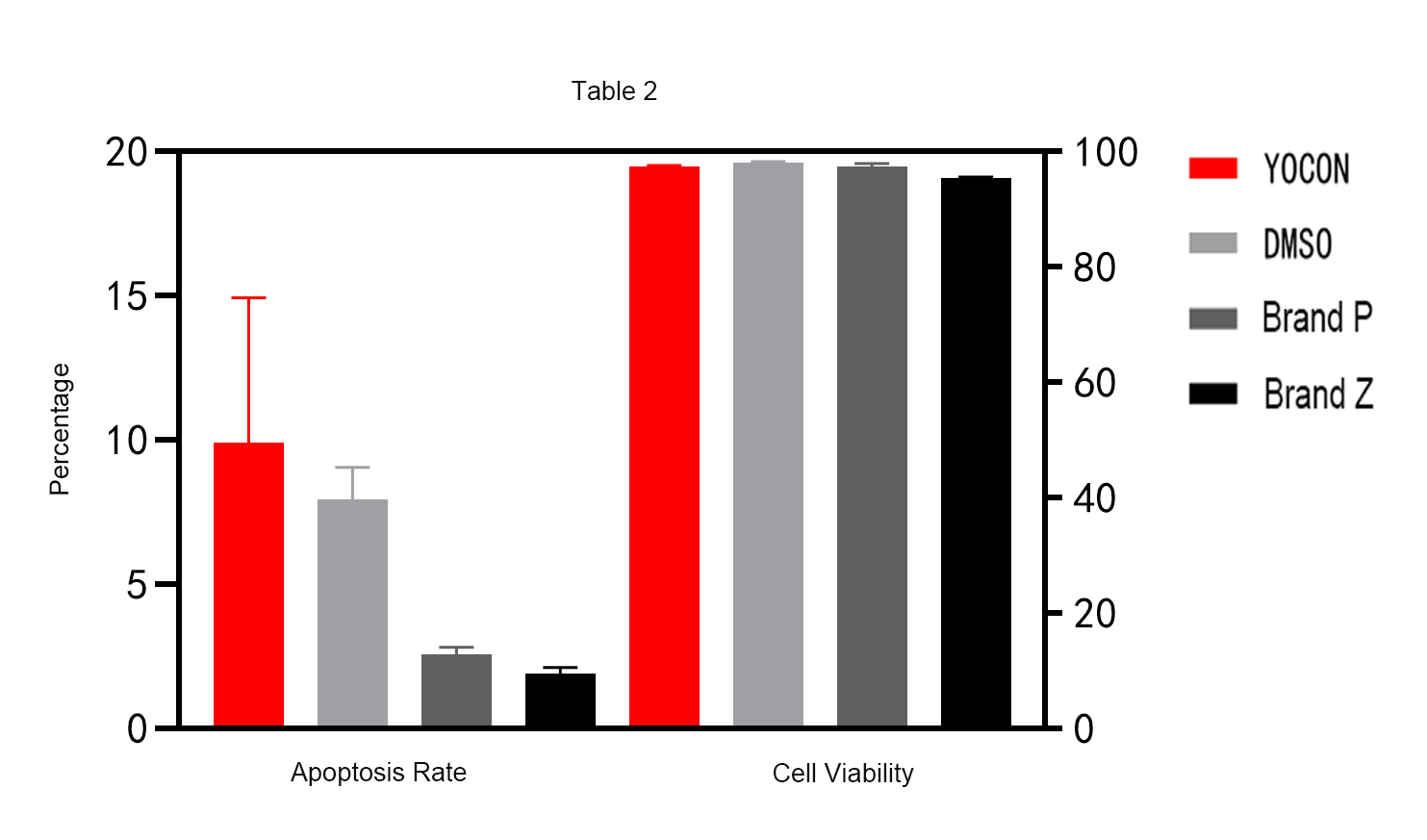
Verification 3 : Compared with the DMSO cryopreservation medium and the imported finished product cryopreservation medium, there is almost no difference in the cell recovery amplification factor of Yocon Biology GMP grade vitrified cell cryopreservation medium.
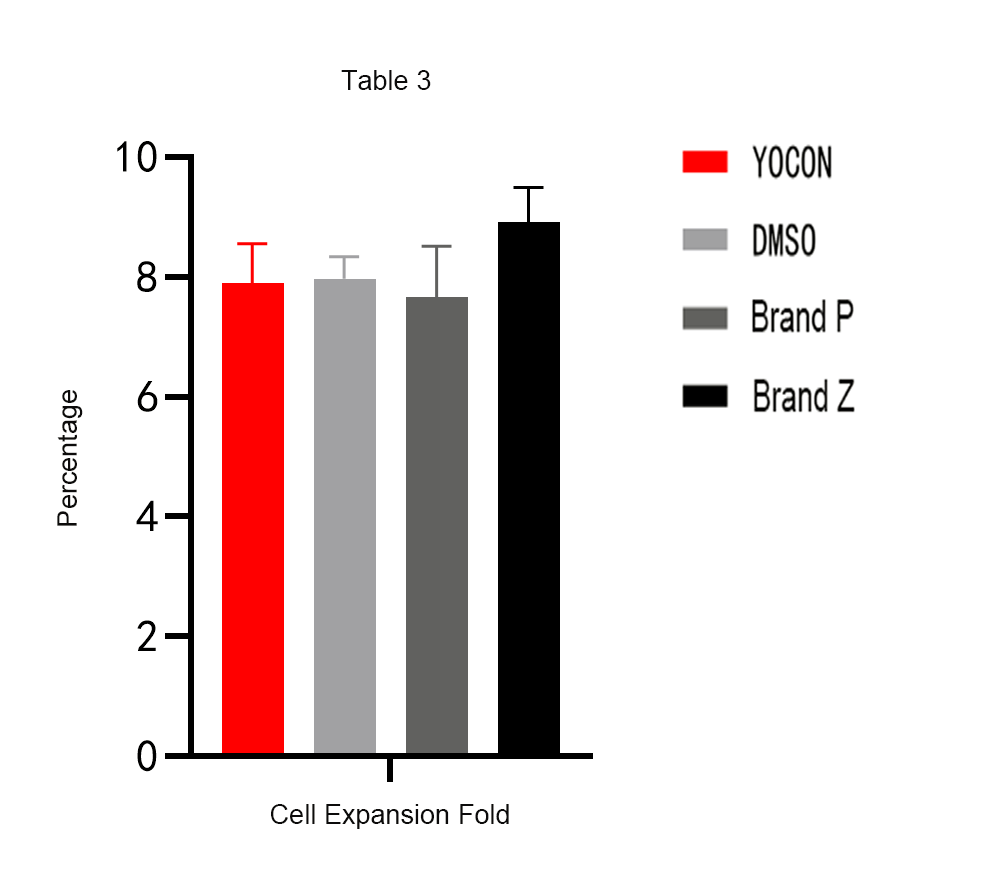
Yocon Biology's GMP-grade vitrified cell cryopreservation medium is truly safe and effective in terms of technology and ingredients. On the technical level, this cryopreservation medium is specially developed for vitrification cryopreservation technology, which can protect the biological activity and basic functions of cells to the greatest extent; in terms of composition, this cryopreservation medium is protein-free, DMSO-free, and animal-derived The ingredients, all the ingredients are clear, produced on a GMP-level production line, which minimizes safety risks and meets the safety requirements of drug declaration users.
With the continuous development of the cell industry, users' demand for products is also constantly upgrading. In order to better meet the needs of users, Yocon Biology will continue to innovate and provide users with products with better performance and more convenient use.

 Yocon Biology
Yocon Biology 2023-06-29
2023-06-29 Company Events
Company Events